Chemical interaction between aluminium and food
In this article, starting from basic chemistry, the subject of the interaction between aluminium and foodstuff is tackled, providing numerical data the result of experiments carried out with the greatest stringency. It is confirmed that Italian regulatory legislation on the subject, ratified by DM 76 dated 18/04/2007 safeguards consumers and provides clear indications to avoid improper use. We hope that other European countries will follow Italy’s legislative example. Ciro Sinagra

Aluminium, as a chemical element with the symbol Al in the periodic table of elements, is not naturally occurring.
It is obtained by electrolysis of alumina (or aluminium oxide, Al2O3, a compound that is abundant and naturally occurring, being the third most common compound in the earth’s crust) in Na3AlF6, sodium hexafluoroaluminate, with a major energy expenditure (16 kWh/kg approximately): it is not simple to separate aluminium from its oxide.
Elementary aluminium is amphoteric (it can react both as an acid and a base, depending on the reaction in which it is involved), with a very high response capacity - low ionization energy - and it immediately tends to combine with atmospheric oxygen to become again, in few minutes, oxide (Al2O3); in a water-based solution, it hydrolyses and becomes hydroxide (Al(OH)3) or reacts with acids to become, at least partially, a salt.
It means that, to start with, talking about aluminium migration is not completely right: we should be talking about migration of aluminium ions and of aluminium compounds already occurring on the metal surface (oxide) or compounds that form out in contact with the reaction environment. When, through a chemical analysis (for example carried out by atomic absorption or ICP) we want to measure Aluminium, actually we measure the quantity of Total Aluminium, namely also the aluminium included in any of its compounds.
ABOUT THEORIES
A study carried out by P. Dumas, J. Dubarry-Barbe, D. Rivière, Yves Levy and J. Corset about kinetic growth of aluminium oxide at room temperature [1] shows that it takes only 10 minutes for aluminium to get coated with a protective oxide layer between 2 and 4 nanometres thick, a layer that naturally occurs on the surface of any aluminium product (figure 1).
Oxidation of aluminium at room temperature and standard pressure has been studied through Attenuated Total Reflectance (ATR) tests. In particular, the trend of the minimum angle of reflection, θmin, over the time of air exposure is reported.
As clearly shown in figure 1, after an exposure of approximately 10 minutes, the angle value tends to stabilise, which corresponds to the formation of approximately a 3 nm aluminium oxide on the surface.
Thus, we find some oxide on the surface of any uncoated product in aluminium or its alloy.
This oxide, within a specific pH range (Pourbaix Diagram, figure 2), protects aluminium against corrosion.
When the metal is in contact with very acidic or basic pH environments, this oxide is not able to protect on its own the metal substrate from corrosion that solubilises and forms out compounds that depend on the reaction environment.
In particular, for pH 3+ ions, that then binds with complexing agents occurring in a given foodstuff to form out complexes (citrates, lactates, acetates) [2].
High purity aluminium (99.90%) does not have enough mechanical properties for the production of aluminium trays or foils, usually produced instead with aluminium alloys for plastic forming.
Metals other than aluminium may increase corrosion with resulting galvanic responses. Almost all chemical elements used as alloying elements to produce aluminium alloys have an electro-chemical potential higher than aluminium itself: with water, oxygen and electrolytes, they result in galvanic responses that include energy and matter transport. Being less noble, aluminium reacts as a sacrificial element and corrodes.
For example, magnesium is one of the few elements with a lower electrochemical potential than aluminium and thus, it oxidises quicker, increasing the protection of aluminium against corrosion:
Mg2+ + 2e- → Mg -2,37
Al3+ + 3e- → Al -1,66
On the contrary, iron has a higher potential and aluminium will be the sacrificial element:
Fe2+ + 2e- → Fe -0,44
Laboratory trials: migration testing on laminates of several aluminium alloys in sodium chloride and citric acid (ICP and SEM analysis)
Corrosion (as well as release/migration) of aluminium in a very acidic environment has been studied for both the different aluminium alloys used and putting in contact the metal with a number of foodstuffs.
Usually, several acid and salty foodstuffs, especially those with a long required shelf life, are packed and stored in packaging of aluminium coated with polymer films: just thinking about beverage, meat or fish cans, semi-rigid containers for jam, compotes and fruit purée, etc.
One of the studies has also been carried out in the Laminazione Sottile SpA R&D lab, through release tests on laminates of several uncoated aluminium alloys in contact with water-based solutions of sodium chloride (3.5%w) and citric acid monohydrate (1.0%w, pH = 2.01) at room temperature from 3 to 72 hours.
The analytical techniques used - Inductively Coupled Plasma (ICP) and Scanning Electron Microscopy (SEM) - have shown that:
- up to 24 hours of contact with testing solutions result in moderate attacks on metal (figure 3);
- the higher the purity of metal is, the less aggressive is the attack of solutions;
- sodium chloride develops pitting (figures 4a and 5a); citric acid leads to filiform corrosion along the grain boundaries (figures 4b and 5b).
Figures 4 and 5 show the different corrosion behaviour of the alloys in acidic and saline environments: with the same conditions, an alloy 1200 has a better performance than an alloy 8006.

Figure 1 and 2 (click to enlarge)
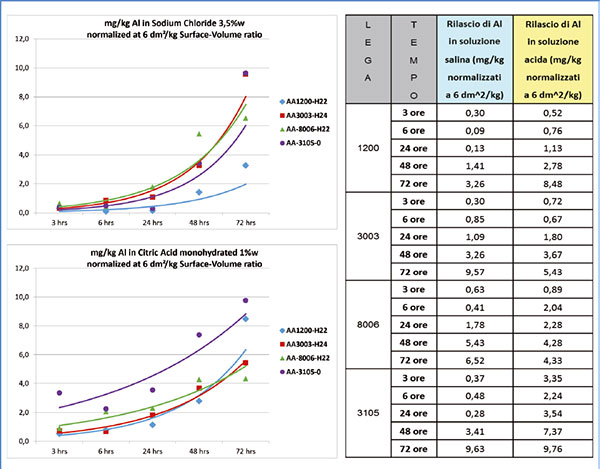
Figure 3 (click to enlarge) - ICP tests on several aluminium alloys in sodium chloride and citric acid at room temperature.
Refutation, according to the data
Thus, it is necessary to underline that carrying out tests under risk conditions, as reported by Bfr in “BfR-Forschung: Nachweis des Übergangs von Aluminium aus Menüschalen in Lebensmittel” of 29/05/2017 [3] does not result in any relevant discovery or innovation: food used for tests are standards above the use limits established by DM76 Decree of 18/04/2007, reported by all Italian manufacturers of food quality grade aluminium trays and foils [4]: on the contrary, no specific law is currently envisaged in Europe. For this reason, these tests are not a proof of neither the accumulation nor the toxicity of aluminium, since the quantity of migrated aluminium found does not correspond with the actual use of aluminium trays at issue: no container in uncoated aluminium can be intentionally manufactured for long-time contact with acidic foodstuff.
It is clear that the pH of a tomato purée is far more critical than some pasta dressed with the same sauce and the aluminium release values will be completely different.
In Italy, studies and tests carried out by Istituto Superiore di Sanità (National Institute of Health), among the first public labs to be interested in aluminium used as food contact material and product (FCM), led the Ministry of Health to regulate the matter through the Ministerial Decree no. 76 of 18.04.2007, a regulation that establishes the recommended rules for aluminium (Annex 1) and aluminium alloys (Annex II) materials and products for food contact.
This Decree established the appropriate use conditions, also as for time and temperature (refrigeration) of use, for consumer protection. Annex I, “Purity requirements for aluminium”, refers to 99% pure aluminium, not used that much in food packaging production due to its low mechanical properties; Annex II, “Composition characteristics of materials and products of aluminium alloys obtained by plastic forming”, refers to aluminium alloys, generally used for food contact aluminium trays and foils.
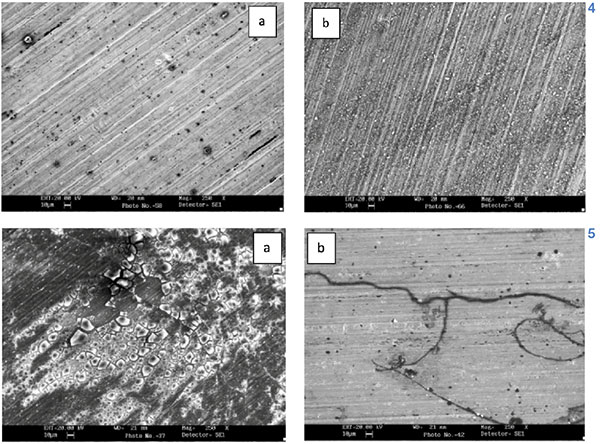
Figure 4.a (Click to enlarge) pitting resulting from sodium chloride on alloy AA1200 H22 after 72 hours; b) filiform corrosion along grain boundaries resulting from citric acid on alloy AA1200 H22 after 72 hours
Figure 5.a(Click to enlarge) pitting resulting from sodium chloride on alloy AA8006 H22 after 72 hours; b) filiform corrosion along grain boundaries resulting from citric acid on alloy AA8006 H22 after 72 hours.
Food tests: aluminium trays and foils for food packaging
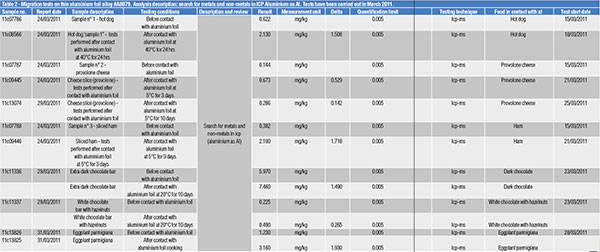
A study, published in 2014 by R. Bonacina and C. Sinagra and carried out on aluminium foils and trays used to cook and store a number of foodstuffs, quantified and distinguished the quantities of aluminium already occurring in the foodstuffs examined from those “released” by the packaging[7].
The values of aluminium trays
(table 1) and foils (table 2) found by ICP analysis are reported.
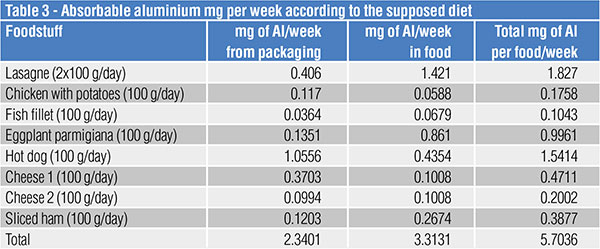
Once aware of the total aluminium quantity, expressed in mg/kg, a 4500 kcal/day diet made up of food cooked and stored only in aluminium packaging has been supposed.
The above-mentioned very extreme hypothesis that has no reference to the actual use of aluminium packaging led to what follows in table 3.
In a week, total absorption was 5.7 mg.
EFSA, in its study “Safety of Aluminium from dietary intake: Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Food Contact Materials (AFC)” - published in the EFSA Journal 2008 754, 1.34 - suggested not to exceed 1 mg/kilo body weight per week (TWI)[8].
It means that an adult with a body weight of 60 kilos should not intake from dietary products more than 60 mg of aluminium per week.
The value of 5.7 mg/week, namely the total amount resulting from the absorbed aluminium of the supposed “extreme” diet, is considered all safe.
Aluminium compounds found in food and drugs
Aluminium Oxide Al2O3
It is a ceramic oxide with a fusion point of 2050 °C, density 3.94 g/cm3 and very low water solubility (approximately 0.001 g/l at 20°C).
Being the third compound in the Earth’s crust (after examining 1672 different types of rocks, F. W. Clarke named it second, after silica SiO2 , establishing a weight percentage of 15.41%), human exposure to it is very high [5].
If aluminium oxide was a real hazard to human health, we should decide, as humankind, to “emigrate en masse” to another planet, a more welcoming place than the Earth (providing it exists)!
Aluminium oxide is used in many applications: dialysis, dental cement, abrasive in toothpaste, wetting agent in food additives. It can be also found in some topical formulations, such as anti-acne preparations.
Al2O3LD50 >5000 mg/kg body weight in rats orally
Aluminium Hydroxide Al(OH)3
Aluminium in water is hydrolysed and forms out aluminium hydroxide.
2Al + 6H2O → 2Al(OH)3 + 3H2
Aluminium hydroxide is commonly used in drugs and can be found in antacids (Alu-cap, Gaviscon, Pepsamar, Maalox etc.) to react in your stomach to reduce the acidity value and, consequently, the risk of ulcers, heartburns and dyspepsia.
Aluminium hydroxide precipitates are used as adjuvants in some vaccines to prevent some proteins from precipitation and accumulation on the bottle walls during the preparation storing.
Al(OH)3 LD50>5000 mg/kg body weight in rats orally
Aluminium Chloride AlCl3
It is obtained after a reaction of aluminium with hydrochloric acid:
2Al + 6HCl = 2AlCl3 + 3H2
Aluminium trichloride is also used in the preparation of topical drugs (epidermis), styptics and antiperspirants. It is used in the treatment of plantar, palmar and axillary hyperhidrosis and bromhidrosis, as well as in the treatment of the athlete’s foot to foster and, consequently, increases the specific antifungal drug effectiveness.
AlCl3 LD50 = 3311 mg/ kg body weight in rats orally
Aluminium Acetate Al (CH3COOH)3
Aluminium acetate is obtained by a chemical reaction between aluminium and acetic acid:
2Al + 6CH3COOH = 2Al(CH3COO)3 + 3H2
Aluminium acetate has astringent and antiseptic properties performed against bacteria, fungi (for example in the athlete’s foot) and viruses (for example in the treatment of Herpes Zoster vesicles); moreover, it can be used for eczema or insect bites.
Al(CH3COO)3 LD 50 = not available
Aluminium Sulphate Al2(SO4)3
It is obtained by a reaction between aluminium and sulphuric acid:
2Al + 6H2SO4 → Al2(SO4)3 + 3SO2 + 6H2O
It is used in pharmacology as local haemostatic for blood coagulation and capillary constriction; antiperspirant, since it reduces sweating due to the constriction of skin pores; decongestant, to reduce blood supply of mucosa.
It can be found in food additives, such as: E520 Aluminium Sulphate,
E521 Sodium Aluminium Sulphate, E522 Potassium Aluminium Sulphate, E523 Ammonium Aluminium Sulphate.
All the above-mentioned products are synthetic and originate from aluminium: they are in the form of transparent crystals or white crystal powder, easily soluble in water and insoluble in ethanol.
They are used to foster precipitation of proteins, for example during the beer production process.
Moreover, they foster the strengthening of vegetable structure during its processing.
They can be found in a number of foodstuffs, such as egg white powder, crystallized and candied fruit and vegetables.
Al2(SO4 )3LD50 = 980 mg/kg body weight in rats
Aluminium Lactate C9H15AlO9
Aluminium lactate is obtained by a reaction between Aluminium and lactic acid:
6CH3CH(OH)COOH + 2Al → 2[CH3CH(OH)COO]3Al + 3H2
In particular, when aluminium is in the form of hydroxide:
3CH3CH(OH)COOH + Al(OH)3 →[CH3CH(OH)COO]3Al + 3H2O
In drugs, aluminium lactate is used as active principle in treating minor superficial infections and irritations of the oropharyngeal cavity mucosa.
C9H15AlO9 LD50 = 540mg/kg body weight in rabbits
Aluminium Citrate C6H5AlO7
It is obtained by a reaction between Aluminium and citric acid.
2C3H4OH(COOH)3 + 2Al → 2[C3H4OH(COO)3]Al + 3H2
As for acetate, aluminium citrate has astringent and antiseptic properties and is administered topically.
A number of studies on neurotoxicity have been carried out on rats in the first weeks of their life, especially on their behaviour, after a constant oral consumption of aluminium citrate for several weeks, but unambiguous interpretations about the related risks have not been provided yet.
Aluminium (as total content of all its compounds) in food
Chemical analysis of a number of foodstuffs allows finding different contents of total Aluminium that “naturally” occur in them.
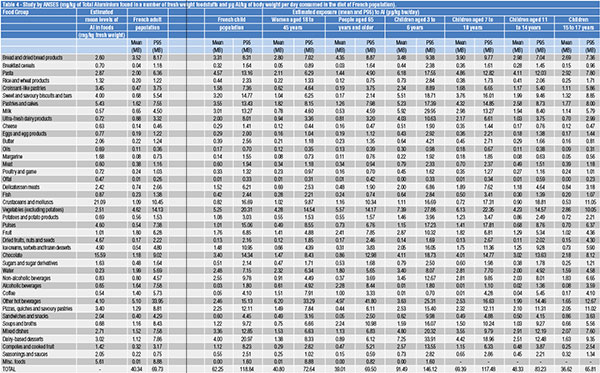
Table 4 reports some data from a French study carried out by ANSES (French Agency for food, environmental and occupational health & safety)[6] to describe mg/kg of total Aluminium found in several fresh weight foodstuffs and µg Al/kg of body weight per day consumed in the diet of French population, divided as follows:
- adults (average);
- children (average);
- women aged between 18 and 45;
- people aged 65 and over;
- children aged between 3 and 6;
- children aged between 7 and 10;
- children aged between 11 and 14;
- young people aged between
15 and 17.
CONCLUSIONS
Aluminium oxide that naturally occurs on the surface in contact with the air with quick chemical kinetics is protective in some pH ranges.
For very acidic (8.7) pH, oxide is not able to protect metal from corrosion on its own and, with different mechanisms according to the substance (for example: sodium chloride or acetic acid develops pitting, citric and lactic acid leads to filiform corrosion along grain boundaries), Al+3 ions go into solution.
However, these ions partially react with the complexing agents occurring in that food, forming out oxides, hydroxides and salts.
An analysis of “migration or release” carried out with atomic absorption techniques or ICP assesses total aluminium, that is especially occurring in the form of compounds.
In order to measure the actual exposure of population, it is necessary to carry out an analysis that, starting from a reference diet (according to the age range, gender and food habits that may vary in different countries) assesses the quantity of total Aluminium already occurring in food and adds the “migrated” amount resulting from the metal packaging.
Only researches carried out under these conditions may assess in a reliable way if we are within the limits suggested by EFSA as for dietary total Aluminium assumption.
In addition, migration or release tests with liquids may also not be significant since they do not represent the actual food contact with the metal holder. Obviously, for very acid liquids, aluminium corrosion has to be expected with migration values close to those reported in the above-mentioned experimental study; the event even gets considerably worse in case of very high temperatures and/or exposure time that exceeds 24 hours.
Italy with its Ministerial Decree n.76 of 18.04.2007 issued some relevant guidelines that led manufacturers to supply consumers with clear use instructions: an Italian producer of aluminium or of its products, designed for food contact, cannot exempt from writing use conditions established by the Decree, thus limiting health risks for an over-exposure through diet.
Moreover, the instructions that the Ministerial Decree n.76 establishes to include in the packaging for aluminium trays and foils have been designed by the Lawmaker to be in a plain language, with no technical terms to ensure the consumer understands quickly and clearly.
It would have been recommended that the Italian statutory approach had been incorporated and applied by other European countries that have no specific regulation (except for France with its obsolete Arrêté du 27 août 1987). The approach taken by BfR [3] is quite questionable: any packaging used in an indiscriminate way may induce excessive migration of substances from packaging to food (imagine how many substances a plastic holder used at a temperature far higher than that envisaged for its use would make migrate to the food). The Nehring Institut, an appreciated and well-known German centre that has always been a European reference point in food contact, also expressed doubts about the BdR approach.[9]
Ciro Sinagra
Laminazione Sottile Group, Caserta (I)
|
BIBLIOGRAPHY [1] P. Dumas, J. Dubarry-Barbe, D. Rivière, Yves Levy, J. Corset - “Growth of thin alumina film on aluminium at room temperature: a kinetic and spectroscopy study by surface plasmon excitation”, Journal de Physique Colloques, 1983 [2] E. Deltombe, M. Pourbaix - “Comportement électrochimique de l’aluminium. Diagrammes d’equilibres tension-pH du système Al-H2O à 25 °C”, Rapport technique R.T. 42 of CEBELCOR, December 1955 [3] Bfr - “BfR-Forschung: Nachweis des Übergangs von Aluminium aus Menüschalen in Lebensmittel”, 29/05/2017; http://www.bfr.bund.de/en/press_information/2017/21/bfr_research_proof_o... [4] Decreto Ministeriale n. 76 del 18.04.2007 - “Regolamento recante la disciplina igienica dei materiali e degli oggetti di alluminio e di leghe di alluminio destinati a venire a contatto con gli alimenti.” [5] F. W. Clarke -“Data of Geochemistry”, 1908 [6] ANSES - Second French Total Diet Study (TDS 2), Report 1: “Inorganic contaminants, minerals, persistent organic pollutants, mycotoxins and phytoestrogens”, Expert Report, June 2011 [7] Sinagra C., Bonacina R. - “Vaschette e foglio di alluminio per l’imballaggio alimentare”, ItaliaImballaggio 10/14 http://www.dativoweb.net/it/contenuti/carcano-antonio-gruppo-laminazione... [8] EFSA - “Safety of Aluminium from dietary intake: Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Food Contact Materials (AFC)”, EFSA Journal 2008 754, 1.34 [9] Institut Nehring - Stellungnahme - BfR - Untersuchung zum Übergang von Aluminium aus Menüschalen auf Lebensmitte l- 10. Juni 2017 - Ref. NENE04-67 - Dr. Ulrich Nehring |