IBSA thought
What does the pharmaceutical industry want from packaging and packaging machine suppliers? A lot, according to Enrico Gasperotti, Head of Manufacturing of IBSA Institut Biochimique, but nothing impossible from a technical and technological point of view, also from an Industry 4.0 perspective. What counts rather is the flexibility of the supplier and their readiness to find the best solutions... And to achieve this goal it is still the people that make the difference. Stefano Lavorini

Traditionally IBSA is able to rely on an excellent production quality. They make most of their pharmaceutical products internally: the active ingredients are made by their affiliates and the excipients and packaging materials are purchased in Europe.
All products obviously comply with GMP standards and suppliers are subject to systematic checks, with particular attention to quality and respect for the environment, as well as in accordance with specific regulatory requirements.
The objective is indeed to implement vertically integrated processes for the control of all production phases, with direct management of the various processing levels and a reduction of risks along the supply chain.
Also with regard to the machines, the packaging and packaging lines, the design and engineering are done in-house, choosing the best that is available on the market and structuring direct partnerships with the various suppliers.
This is testified by Enrico Gasperotti, Head of Manufacturing of IBSA, who I met in the company’s historic headquarters, a sober, almost period building with green shutters, a monument to how much has changed over the years.
IBSA produces products for a wide range of therapeutic areas: what does this mean in terms of production capacity?
Every year, 14 million vials of hormones, 300 million soft capsules and 60 million packs of finished products leave our plants in Switzerland, Italy and France.
We are a purely manufacturing company. In Switzerland we have seven factories, the result of the rapid growth of these years, but we are now planning to build a “common house” in Grancia, with a total investment of 80-90 million francs, highly substantial hence.
All the same our packaging lines comprise machines from different manufacturers, including IMA, Marchesini, CAM, Etipack...
All key names for the pharma sector; but for you what makes the difference when you have to make purchasing choices?
We have always found great attention to our needs in Italian manufacturers, that is the possibility of developing new projects with our partners: in the pharmaceutical sector, moreover, the processes are very varied and complex and the packaging - whether its tubes, cases, blisters – is always different in shape, weight and technical characteristics.
The success of a project is nevertheless based on exchange and dialogue and, in this sense, we find differences between our various suppliers. Once, perhaps, it was simpler because, today, many concerns have a more complex organization, with procedures that, for example, allow you to monitor job progress, but something has been lost in terms of flexibility. That’s inevitable.
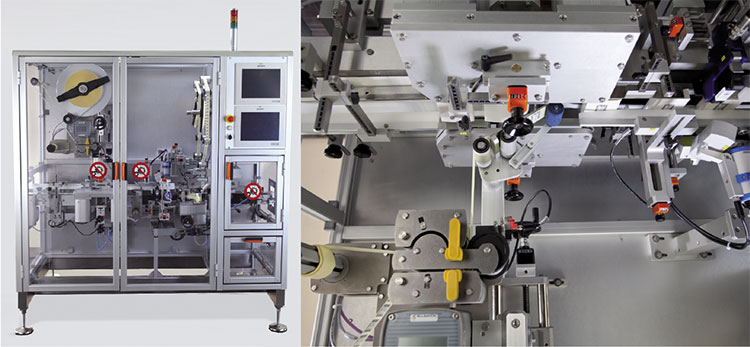
Pharma 4 by Etipack for Track & Trace in line. A single automatic traceability and pharmaceutical serialization, labeling (application of pharmaceutical stamps/vignettes/label for serialization in China) and anti-counterfeiting system, with the application of two self-adhesive labels on the flaps of pharmaceutical cartons (full and closed boxes).
It seems clear to me that you prefer not to delegate the realization of the solutions you need to a single supplier...
We are constantly expanding and, often, we have to build new sections to produce new products. This is perhaps why design, engineering and development are done by drawing on internal expertise, something what is more that we intend to consolidate and increase.
I believe that, where management knowledge and knowhow and the willingness of people to take on responsibility for technical choices diminishes, this is to the detriment of the company’s management and decision-making skills.
For us, a certain stability in relations, in human relationships, an aptitude for dialogue, the determination to achieve the result.... these remain fundamental... All things we do not want to give up on.
The pharmaceutical industry is called upon to face constant new challenges in terms of patient safety and protection: first segregation, now serialization... Have you had to make significant investments in this sense?
These are very concrete aspects that, in this specific case, we have faced with the aid of Etipack, our traditional supplier for over 30 years, with whom we have excellent relations.
Together we have worked on the installation of solutions tailored to our needs, such as serialization and track & trace systems.
As mentioned, we do not buy complete lines, but the machine we need, from whom we consider the best manufacturer. Inevitably, having to link up the machines in line anyway, we require that the various builders dialogue with each other: and in dealing with this aspect we have always found great readiness on the part of Etipack, to whom, moreover, in general terms, we acknowledge a more than satisfactory after-sales service. In short, Etipack has never left us to tackle a problem on our own.
Industry 4.0: what are you doing about it? Very often, in this area, there is talk of predictive maintenance: do you feel that need?
For over two years we have been investing in IT systems able to manage the activities concerning digitization of production.
To date, however, we are not planning to implement predictive maintenance systems, also because for several years we have adopted a precise program of preventive maintenance of the lines, with highly interesting returns: for example, we have almost zeroed sudden stops, ie the unmanaged ones, which have a great impact on productivity
On this regard it should be noted that, in Switzerland, work permits for foreigners are regulated by quota; practically speaking in Switzerland, an Italian manufacturer has a maximum of ninety days/year for interventions related to installation and assistance. For this reason, some concerns have set up companies in Switzerland, with resident technicians to remedy the problem. Many but not all.
How do you see the future of the pharmaceutical industry?
IBSA is constantly growing, as is pharmaceutical production in general. Italy is now Europe’s leading producer of pharmaceutical products (IBSA has among others a large production plant in Lodi and one in Avellino, Ed.).
Hence the outlook is good, considering on the one hand the progressive aging of the population in the industrialized countries and, on the other, access to health services of new segments of the population in emerging countries.
However, one needs to preside over the right therapeutic seam - like, for IBSA, that represented by pharmaceutical products for the treatment of thyroid dysfunction - also because there is a true and proper price war being waged by all the Health Authorities to keep health expenditure under control.
To the point where now some products are sold almost at cost price: The margins are continually decreasing, while the costs of activities that are not strictly associated with production but nevertheless related to it, such as development, innovation, but also the improvement of existing pharmaceutical products, require huge investments.
In short, you have to be evermore efficient to stay on the market.
Caring Innovation
Quality, innovation, people, responsibility: these are the four pillars of IBSA Institut Biochimique SA, a Swiss pharmaceutical company founded in 1945 by a group of biologists. Its specific identity was however defined in more recent times, in 1985 when it was bought by the current owner, who redefined its strategy by focusing on the development of technologies capable of improving the existing therapeutic solutions qualitatively. From this fundamental stage, a dynamic development program has been launched aimed at consolidating the global strategy of territorial expansion and extending the company assets, thanks to which IBSA has achieved a leading position on world markets - in specific therapeutic areas - leading to its presence on 5 continents and in over 80 countries.
Today, IBSA is the largest privately owned pharmaceutical company in Switzerland, the fourth largest operator in the “fertility” sector, after the big multinationals, and one of the world leaders in the supply of hyaluronic acid based products.
In August of this year, when the start-up of the commercial activities of IBSA Pharma Inc. were announced in the USA, Arturo Licenziati, Chief Executive Officer of the group, commented: «The decision to directly market levothyroxine in soft capsules in the United States (previously distributed by an American company under license, Ed.) will allow us to maximize its value, as well as introduce new IBSA pharmaceuticals on the market, some of which are already awaiting approval by the FDA. This is an important step, which allows us to look to the future with even more confidence».
A prospect what is more with solid foundations.
The IBSA headquarters are at Collina’Oro, in the immediate vicinity of Lugano, Switzerland. Production plants and laboratories are distributed in 9 other sites located in Canton Ticino, for a total of 360 employees assigned to production and another 200 to collateral activities.
Each plant is dedicated to specific production lines, in compliance with the principle of segregation of production processes: a strategy that has enabled certification by the most demanding regulatory bodies to be obtained, as evidenced by recent approvals of IBSA products in the USA.
Over the years IBSA has also grown a lot outside the Swiss borders: in the world, the company is represented by 10 branches, and today employs 1,400 people, distributed between headquarters, branches and production sites, plus 200 employees of its strategic partner, Laboratoires Genevrier in France.

















