Risk assessment of printed articles marketed worldwide
A brief overview of regulations currently in force in different regions of the world reveals a highly heterogeneous and troublesome situation with which producer companies must contend.
by Marinella Vitulli
RELATED ARTICLE
Food contact materials and printed articles: regulatory trends
EUROPE
In Europe, an object containing printing inks must meet the general compliance requirements listed in Article 3 of Reg (EC) 1935:2004, not releasing any components into the food product or modifying its taste, appearance, texture or aroma.
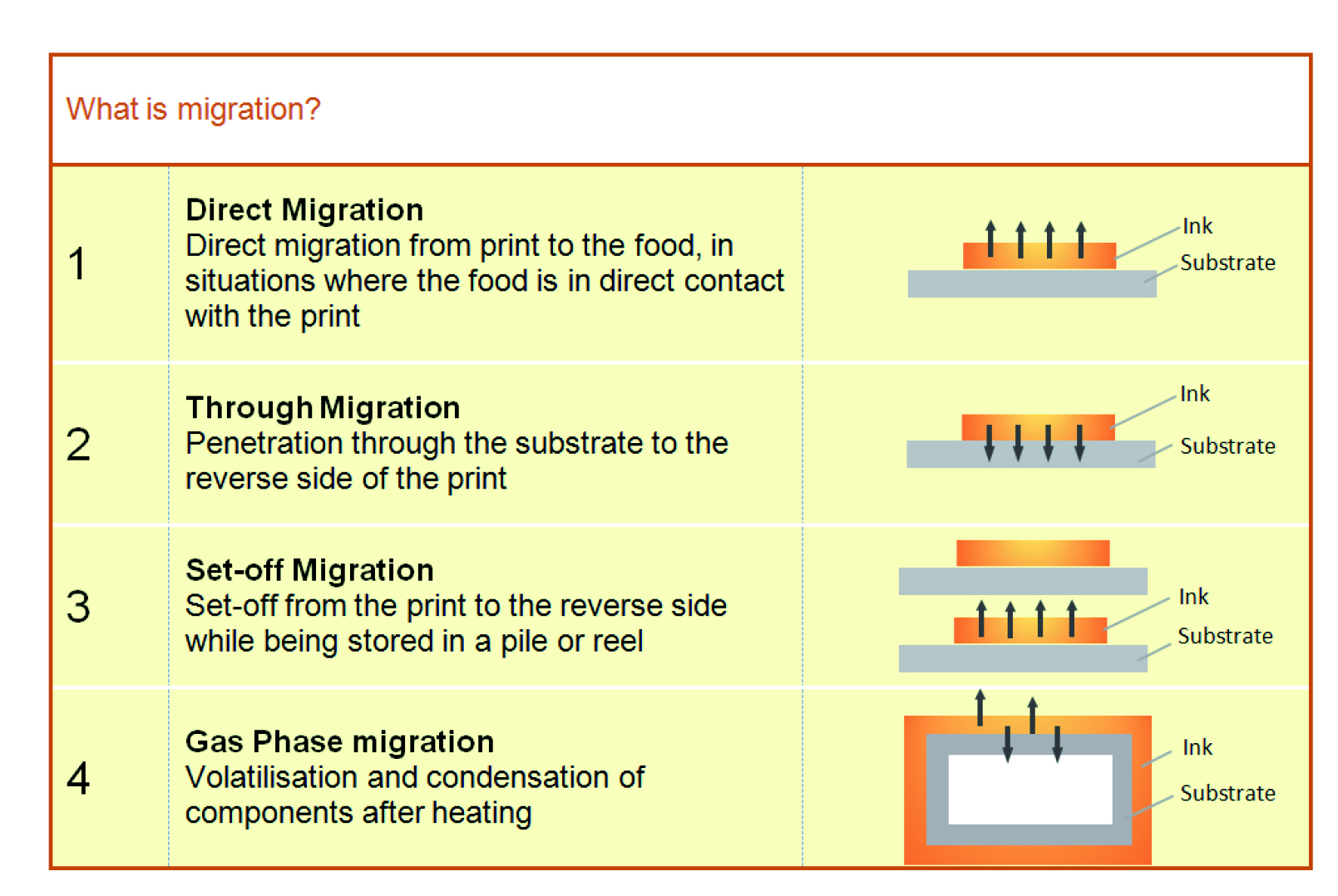
It must also comply with Annex I of Reg (EC) 2023:2006, which stipulates that inks must not be transferred to surfaces in contact with food products by migration through the substrate or due to set-off when stacked or while on reels.
According to the 2016 GMP guidelines of EuPIA (the European Printing Ink Association), migration of ink components can occur in a variety of ways (Fig. 1). At the moment, printed materials must meet various requirements, depending on the countries in which they are marketed. Adequate risk assessment is therefore necessary in order to ensure that printed articles are in compliance with European regulatory frameworks.
Furthermore, although there is still no specific European legislation governing printing inks, printed plastic objects must comply with Regulation (EU) 10/2011, and the presence of any substances contained within inks and subject to specific restrictions must be notified to all actors along the supply chain and analyzed. There are also a number of local laws – such as those in Switzerland – defining a list of acceptable substances, in which each listed component is subject to specific migration limits.
As for Denmark, Finland, Iceland, Norway and Sweden, in 2012 the Nordic Council of Ministers published the document “Food contact materials and articles: Printing Inks”, which contains a variety of information and a checklist for evaluating printing ink compliance.
USA
In the USA, the Food and Drug Administration (FDA) regulates food additives, which are categorized as either direct or indirect. The substances comprising packaging, including printing inks, can sometimes be considered indirect food additives.
For the FDA, food packaging composed of plastic, paper or other materials containing adhesives or coatings are governed by the 21 chapters of the Code of Federal Regulations (CFR), which provide positive lists of allowable components.
However, no such regulations exist for printing inks used for food packaging. Consequently, whenever the component substances of an ink are not specifically FDA-approved, it is necessary to go through authorization procedures, or else demonstrate that a functional barrier exists. The FDA defines a functional barrier as a material that prevents ink components from becoming indirect food additives.
Migration testing and other types of analysis may be necessary in order to prove the absence of migration. For this purpose, the FDA sensitivity limit threshold is 50 ppb.
CHINA
In China, the National Health and Family Planning Commission (NHFPC) has recently published the GB 9685-2016 National Standard for the Uses of Additives in Food Contact Materials and Articles, which went into effect on 19 October 2017.
The standard specifies the requirements for using additives and also provides positive lists for the substances that can be used for inks.
Compared to the previous standard, the title, scope, terms and definitions have been revised.
The positive lists have also been revised, but the list is still not exhaustive. Many ink producers are therefore concerned that the positive list does not include all substances allowable by law in other countries.
SOUTH AMERICA
In South America, printed materials must comply with the rules dictated by Mercosur/Mercosul (in Spanish: Mercado Común del Sur; in Portuguese: Mercado Comum do Sul), the economic union binding Argentina, Brazil, Paraguay, Uruguay and Venezuela.
Mercosur’s expert committees produce so-called GMC Resolutions, which must be incorporated into national legislation in order to be enforced. The relevant GMC Resolution in this case is the framework resolution 3/92, which provides general criteria for all food contact materials. This is no resolution specifically dedicated to inks.
There are currently 27 resolutions on food contact materials, 10 of which concerning plastic materials (including varnishes and coatings). GMC 56/92 provides general criteria for plastic packaging and articles.
Materials used must comply with general and specific threshold values, according to rules similar to those described by Regulation (EU) 10/2011. Resolution 15/10 also regulates the purity of coloring agents applied to plastic materials, which in Europe have already been replaced by a series of migration tests.

















