Food labels: the new EU regulations
EU Regulation 1169/11 names e-commerce and clarifies what (and how) to write in the ingredients list and nutritional table, how to understand place of origin and the responsibility of operators... the new European regulations on food labeling as read by GS1 Italy| Indicod-Ecr.
 In late 2014, EU Regulation 1169/2011 will go into effect, governing the labeling of food products, with the burden of compliance falling on the manufacturing and distribution concerns. The regulation pursues the objectives of simplifying national and European regulations (28 markets), in addition to greater consumer protection, and introduces various significant novelties to the implementation of European directives already in force. But it also presents a few ambiguities (which risk becoming out and out incongruities) and a series of aspects that are still yet to be defined, which the European Commission intends to clarify soon, by the date of implementation at the latest.
In late 2014, EU Regulation 1169/2011 will go into effect, governing the labeling of food products, with the burden of compliance falling on the manufacturing and distribution concerns. The regulation pursues the objectives of simplifying national and European regulations (28 markets), in addition to greater consumer protection, and introduces various significant novelties to the implementation of European directives already in force. But it also presents a few ambiguities (which risk becoming out and out incongruities) and a series of aspects that are still yet to be defined, which the European Commission intends to clarify soon, by the date of implementation at the latest.
This legislation was the topic of discussion at a conference held last November 18th by GS1 Italy | Indicod-Ecr in Milan where a group of experts examined and commented on the basics of the new EU law. Under the magnifying glass were regulatory/juridical and technical requirements in order for labels to comply, but also the necessity of effectively sharing the contents of these within the industry and the tools developed by GS1 for this purpose.
What has resulted is a complex regulatory framework, rich with information and some delicate issues, which will give much to do to brand owners, distributors, designers and operators in the packaging industry, as well as lawyers. Below are a few of the main points of interest in the provision.
Field of application
With the new EU Regulation 1169/11, nutritional safety is given a more prominent role, no longer limited to the packaging of products marketed in retail or ho.re.ca., but also those employed in public projects or destined for remote sales.
In such a way, the good practices of labeling make their appearance also on the stage of e-commerce, a channel experiencing constant growth, and which is also important in Italy, where, according to data cited by Andrea Ausili, it is growing at an average +20% and an (extraordinary) +11% in grocery retail: the attention which the EU Regulation dedicates to it is therefore well justified. This was also shown by the talk given by Alessandra Di Franco of Mondelez: the multinational invests heavily in e-commerce and has dedicated a cross-functional project to virtual labels, aptly named “Babylon” – which involves 33 European countries and all product types, with contributions from all its partners in the food industry. The point of departure for the project is Article 14 of the Regulation: if products are sold via Internet, compulsory information must be available to the consumer before the purchase is finalized. And not only for general decency, but also and especially in order to protect the health of at risk consumers - those suffering from allergies, coeliac disease and much more.
|
From B2B to B2C: “online” labels One of the novelties introduced by the new European regulation on food labeling concerns information on products sold online. Legislators recognize the importance of this channel and propose provisions to govern it for the first time, with the intention of making available also through this channel any news necessary for protecting the consumer’s health and safety, efforts until now having proven inadequate in terms of quantity and quality. For this purpose, GS1 has dedicated an ambitious project called GS1 Source, conceived as a single, reliable source of standard information entrusted to and managed by brand owners. Its most important tool is represented by GS1 standards for the registration, organization and sharing of data used worldwide in the B2B context and now also to facilitate dialog with consumers (B2C). Its necessary supplements are already operational: the platform (TrustIT) and the app ScanIT, for use with a cellular phone. |
As for the context of this new legislation, the new law seeks to harmonize national regulations and at the same time to leave individual countries with complete freedom to integrate articles of the Regulation into their own laws. These, however, should not hinder the free circulation of goods in the single market or discriminate against products made in other countries, unless they serve to protect public health (for example, to prevent the spread of an epidemic... the danger of which bird flu has illustrated). That this is a difficult objective to reach is shown by the various questions still to be cleared up, among them the management of controversial national labeling systems like the UK’s “traffic lights” (images of which quantify the healthfulness of a food product in accordance with the presence or lack of essential nutrients, Editor’s note) and similar such provisions in Northern Europe.
What changes
 The object of this legislation is thus complex, its requirements numerous and the regulation, consequently, has many points to consider.
The object of this legislation is thus complex, its requirements numerous and the regulation, consequently, has many points to consider.
• The listing of ingredients changes and now must indicate allergens more carefully; the addition of water and the nature of vegetable fats and oils must be explained. Here we also find the first definition of “engineered nano-materials”.
• A few delicate issues concerning place of origin (required reading), which remains optional, except where this may generate misunderstandings (and this is new). Still in force, on the other hand, is the obligation to indicate the origin of bovine meat, and this has been expanded to include other meats (but the list is inexhaustive) and foods: after three years, the European Commission will evaluate the real benefit of this.
• Various elements in the nutritional table change. They will now also need to refer to the amounts per 100 g/ml of product. A few exemptions are worth noting, as well as (also in this section) references to national systems and so-called “traffic lights”.
• The acronym GDA (guideline daily amount) has been abolished. However, reference amounts remain, which is to say the substances in relation to average daily recommended intake. These will need to refer to 100 g/ml - Kcal, FOP - and the serving size (which can also be identified using symbols and pictograms).
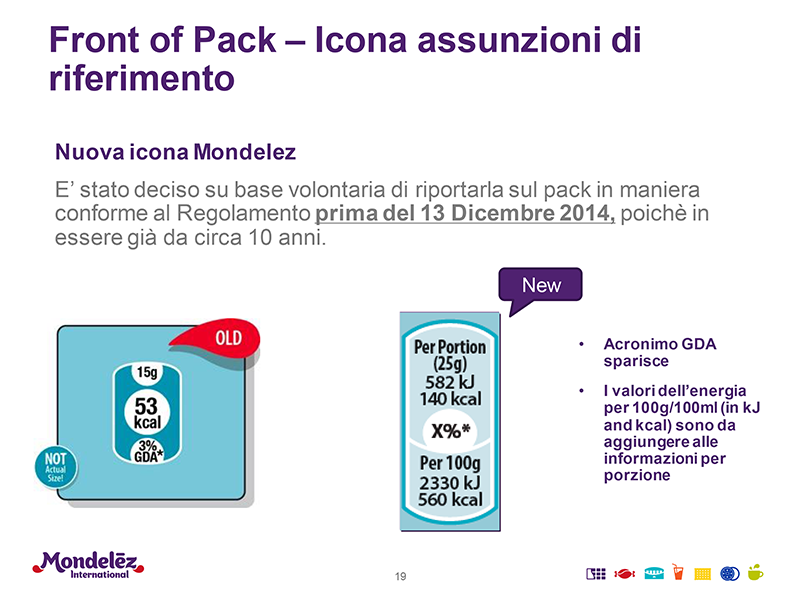 • The graphic requirements for legibility of obligatory instructions relative to available surface are numerous, various and often still to be defined. The most significant novelties include an obligatory expiry date on all pre-packaged single servings.
• The graphic requirements for legibility of obligatory instructions relative to available surface are numerous, various and often still to be defined. The most significant novelties include an obligatory expiry date on all pre-packaged single servings.
• Finally, an essential clarification: whoever places their own name or company name on a food product (the brand owner) is responsible for the completeness and truthfulness of the information reported on the label. For non-EU food products, the importer is responsible.
Note. The accompanying images are taken from a report by Alessandra Di Franco of Mondelez (formerly Kraft Foods), a multinational which has already updated its own labels and is now working on online information.




















