Traceability and biomedical devices: the EU turning point
The integrated systems for inspection, coding and marking for the biomedical devices market were the focus of a training seminar organized on October 12 by Nimax (leader in Italy in the field of coding and marking) and by the Brescia based Antares Vision (global supplier of visual inspection, tracking and intelligent data management systems).
In the location of Villa Quaranta (VR), after the speeches of the promoters of the event to present the state of the art of the sector, experts from leading companies, such as GS1, Romaco and Warrant Group, took their turns to explain to the representatives of the main companies in the medical sector the requirements set by the new European directives, advising them on how to deal with technical, organizational and administrative problems.
The audience showed particular interest in understanding the different aspects of the legislation, from marking and traceability issues on packaging, to the obligation to implement UDI coding on each and every medical product sold or distributed within the EU.
However, it should be borne in mind that, to date, this latter requirement can not yet be met by 70% of the production lines active on the market, and that the average tooling-up time to make a line compliant is over 12 months. Nonetheless, with the entry into force of the new EU Medical Device Regulation (EU MDR), manufacturers and distributors of medical devices have a legal obligation to ensure, by the end of May 2020, that a UDI code (plus any other mandatory information contained in the Annex VI Part B of the regulation) is assigned and registered in EUDAMED* for each product item.
Glossary of EU regulations Medical Device Regulation (EU MDR)
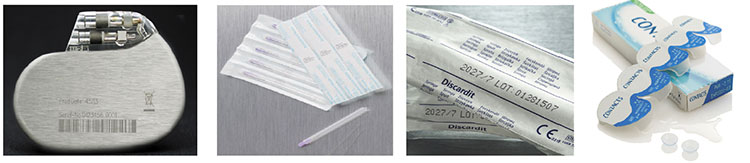
UDI: it is a unique numeric or alphanumeric code that must be affixed to every medical device, label or packaging and must be in a format that can be read by both humans (alphanumeric text) and machines (barcodes or datamatrix).
The UDI code consists of two parts:
1. Device ID (UDI-DI). Identifies the specific version or model of a product (Mandatory and Fixed).
2. Production ID (UDI-PI). It is variable and may contain one or more of the following information:
• Batch or batch number where the device was produced
• The expiration date of a specific device
• The serial number if required for specific devices
*EUDAMED is the European medical device database. Its purpose is to strengthen market surveillance and transparency in the medical devices sector by providing rapid access to information to the competent national authorities.



















