Aluminium foil: focus on the surfaces
SHARING KNOWLEDGE & KNOWHOW Analysis of both sides of an aluminium rolled foil, of which the morphological and chemical characteristics have been evaluated. Resorting to innovative analytical techniques for nanometric and stratigraphic scans of the chemical composition allows us to understand the phenomena that affect the polymeric coating adhesion and any corrosion phenomena. Thus the way lies open to further improvements, to the benefit of converters and users.
C. Sinagra, F. Bravaccino, C. Velotti
Aluminium foil, with gauges between 6 and 40 microns, is widely used both as plain foil (rolls for domestic use, sheets for wrapping chocolate bars) and as a base material for the so-called “converter foil”, a thin foil that is either lacquered or laminated to polymeric films, paper, etc.. The converter foil is widely used in flexible packaging, but also in technical applications (for building insulation) or as a decorative element to enhance the aesthetic appearance of materials. For these micrometric gauges the rolling process is doubled: in practice two foils, from two separate rolls placed on two unwinding reels, are passed through the rolling cylinders. As a consequence, on the winding reel a single coil is obtained, composed of two foils, which are subsequently separated and cut into strips on special lines, known as separators or splitters. The result of this technology is the typical “finishing” of the foil, glossy on one side and matt on the other: the glossy side is determined by contact with the rolling cylinders while the matt side is formed by the contact between the two aluminium films.
The objective of this study is to examine the two surfaces of the rolled foil and evaluate the differences in terms of:
1. surface microgeometry;
2. morphology under the SEM and surface oxygen;
3. surface chemical analysis by innovative GDOS Pulsed Radio Frequency Optical Emission Spectrometry instrumentation;
4. surface energy;
5. measurement of corrosion resistance, by means of potentiodynamic polarization tests.
TESTING

Figure 1. Leica DCM – 3D with scanning unit and underlying inertial insulation substrate.
1. Surface microgeometry study
Description of the equipment used and the tests
The Leica DCM Scan - 3D (figure 1) was the confocal microscope model used for the microgeometric characterization of surfaces (glossy and matt) of the 37 micron EN AW 8079 foil in the annealed physical state.
To ensure maximum reliability of image capture, especially whenever it is prolonged in time and at high magnification, the Leica DCM-3D was installed on a base enhanced by the active vibrations isolation Halcyonics micro system by Accurion. The DCM-3D has two software systems, LeicaScan and LeicaMap, respectively for the management of the different modes and confocal image capture options and for the post processing of the results and analysis of the same.
Both the interchange of the various optics and the optimum acquisition parameters - ie number of layers and the scanning pitch, the type of image capture, the type of light source and references and the start of scanning - were set via the LeicaSCAN software, that can also be used to select the type of image capture.
The most important image captures are Topography, the scanning of a specifically defined area and Extended Topography (Stitching), the scanning an area going beyond the specific one, comprising an area or a set of areas of different shapes “sewn” together to give a defined and settable scanning surface.
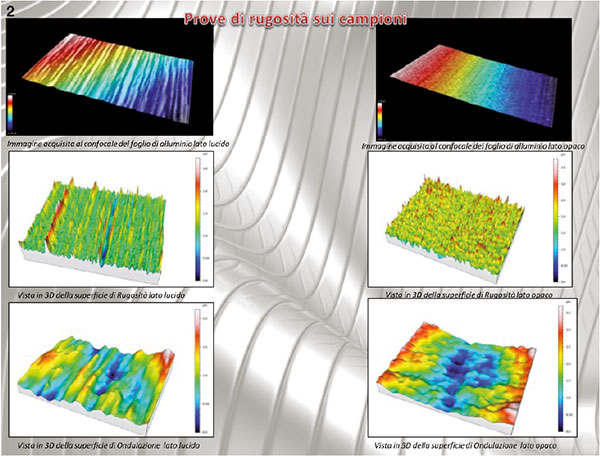
Figure 2. 3D image taken by dual focus microscope: to the left the analysis of the surface’s glossy side, to the left the matt side.
The Extended Topography (Stitching) with areas of about 2mmx2mm was the adopted image capture mode.
20X magnification confocal image captures were carried out, so as to highlight the detailed morphology of the area of aluminium foil under examination.
The captured images were saved and then processed with LeicaMap software, which enables a significant enhancement of the results.
Both the gloss and the matt side (figure 2) of the aluminium foil surface were examined: the obtained images were processed with a specific software, which enhances the quality of the images to obtain the desired data, that are then processed. In this case, after performing a filtering operation, the average roughness in Ra was rated, enabling the surface roughness to be separated from the corrugation of the complete surface. An 80 μm filter was used.
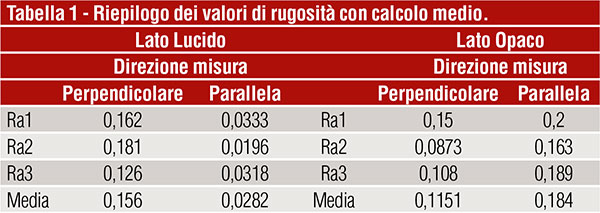
Analysis of the results
Table 1 gives a summary of the measured roughness values. The graphical overview is shown in figure 3, where a big difference in terms of roughness (Ra) can be seen on the glossy side between measurements made parallel and perpendicular to the rolling direction. This difference is not so noticeable on the matt side.
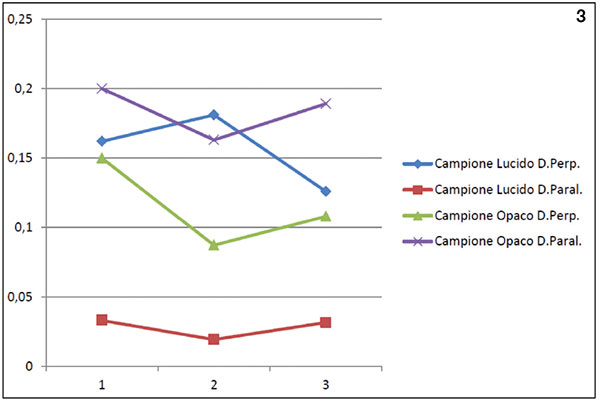
Figure 3. Graph of the Ra values measured on both samples and on the two lamination sides.
2. Morphology under the SEM and surface oxygen
The two surfaces were observed u nder the SEM (scanning electron microscope) to check the morphology, while the EDS X-ray microprobe attached to the microscope was used for the mapping operation, to highlight the aluminium oxide present on the two aluminium surfaces, measuring any differences. It should be stated that the examined sample was taken from an aluminium coil after recrystallization annealing in an industrial furnace (in air) carried out at 280° C for 30 hours.
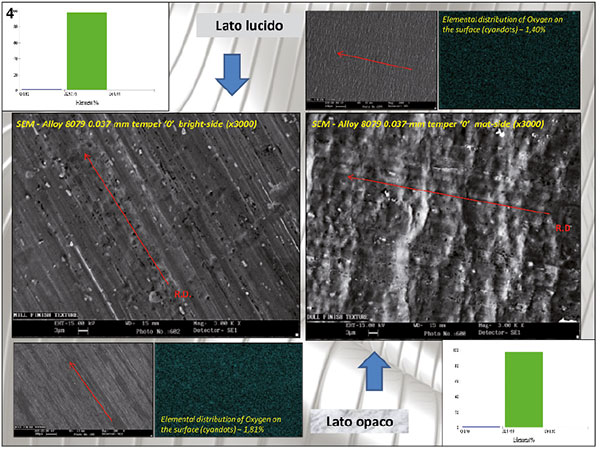
Figure 4. SEM observations: to the left the gloss and to the right the matt side. Mapping of the surface oxygen and determination of the element on the surfaces of the samples. 1.81% of oxygen revealed on the glossy sample, 1.40% on the matt side.
Figure 4 shows the SEM images of the two surfaces, the surface oxygen mapping and the histogram with the oxygen analysis on the two surfaces, showing 1.81% on the glossy surface and 1.40% on the matt surface.
3. Surface chemical RF-GDOS analysis (Pulsed Radio Frequency - Glow Discharge Optical Emission Spectrometry)
Analysis with the EDS probe attached to the electron microscope has the disadvantage of excessive penetration of X-rays into the sample of up to 2 microns, hence the surface analysis was carried out with the GDOS Horiba Scientific spectrometer.
The GD-PROFILER 2 system facilitates fast analysis of solid materials in various application fields, such as coating by PVD/ CVD deposition, coatings, electroplating materials, oxides, ceramics, semiconductors, glass and photovoltaic materials.
Thanks to the simultaneous image capture system integrated to a “Radio-Frequency Glow Discharge” source, ultra-fast analysis could be carried out of bulk surface component and of the deep profile of both conductive and non-conductive multilayer samples.
The deep profiles range from a few nanometres to more than 200 microns and all the elements of interest can be simultaneously analysed.
Furthermore, an Imaging monochromator can be added for the analysis of the full spectrum emitted by the sample, to continuously define the spectral region of the sample analysis.
Figure 5 shows the surface analyses performed on samples with a GDOS spectrometer, on the glossy (green curve) as well as the matt side (blue curve).
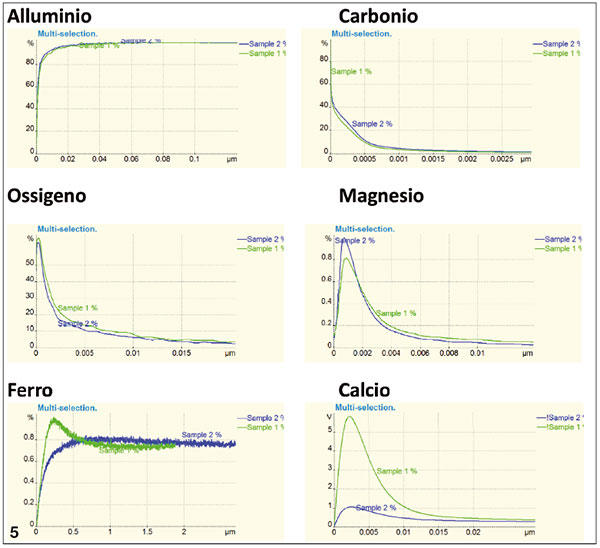
Figure 5. Chemical analysis done via GD OS spectrometer, that enables a nanometric analysis by layers (depth) of the inner sample surface. The green curve shows tests on the shiny side, the blue curve the matt side.
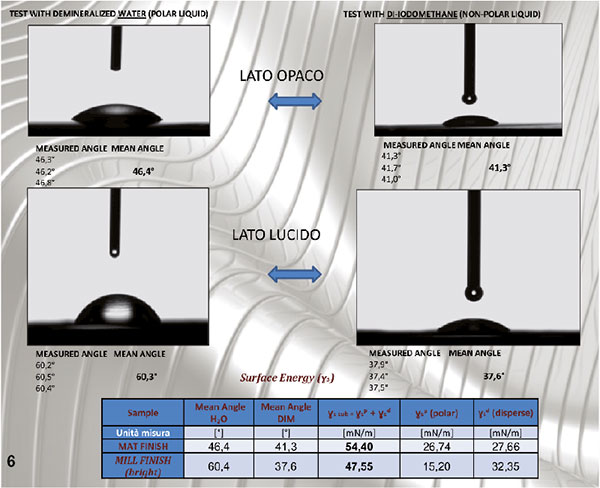
Figure 6. Wettability tests made on the matt side (above) and shiny side (below). The matt side has a higher surface energy than the shiny side (54.4 against 47.5 mN/m)
4. Surface energy measurements
The study was carried out by performing wettability tests with high polarity liquid (water: polar phase 51 dispersed phase 21.8) and very low polarity one (di-iodomethane: polar phase 2.3 dispersed phase 48.5) by depositing drops of water of 8 µl and iodomethane of 1,9 µl on the samples. The surface energy was calculated according to Owens-Wendt and the values expressed in mN/m..
Figure 6 shows the summary of wettability tests and measure of surface energy.
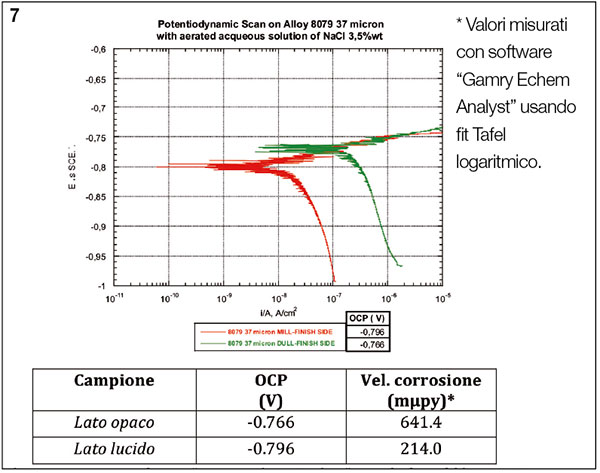
Figure 7. Potentiodynamic test in watery solution aerated 3.5% with sodium chloride. Tests carried out on the matt (green) and gloss (red) side.
5. measurement of corrosion resistance, by means of potentiodynamic polarization tests.
To evaluate the corrosion resistance of the glossy and matt surface on the same aluminium foil, electrochemical measurements were carried out using the potentiodynamic polarization curves using an electrolytic solution of Sodium Chloride weighing 3.5% (figure 7). The tests were performed with a Gamry Reference 600 system with the saturated calomel electrode (SCE) as a reference, and a platinum one as an electrode counter.
The polarization range was set to ±0.200 V compared to the open circuit potential (OCP) and a scan of 0.1667 mV/sec.
The potentiodynamic polarization curve shows a better resistance to corrosion of the gloss side (the estimated corrosion rate is of 214 nanometers per year, compared to 641 of the matt side). This performance can be due to the presence of a greater amount of oxygen (Al2O3) on the glossy side, also found in the EDS and GD-OES tests, that renders the surface passive and more resistant to chemical attack from the sodium chloride solution.
SUMMING UP
1. The roughness tests show Ra values very different on the gloss side of the rolled foil, between the measurements made parallel and perpendicular to the rolling direction. These differences are not found on the matt side.
2. Examining the controls made under the SEM via an xray EDS microprobe, the gloss side is seen to have an oxygen percentage higher than the matt side.
3. The Pulsed RF-GDOES analysis confirms a slightly higher oxygen content on the gloss side. The presence of carbon indicates traces of cracking of the lubricant used in the rolling process, formed during the final annealing heat treatment. This presence seems to be higher on the matt side, but does not cause a deterioration of the wettability. The magnesium, although present in very low concentrations in the alloy (only 8 ppm) totally migrates to the surface and appears to have its maximum peak concentration at 1 nanometer.
4. The surface energy evaluation tests show that, with equal thermal treatment, the strip has a better “wettability” on the matt side compared to the gloss side (average value = 54 mN/m matte side, 47mn/m gloss side).
5. The potentiodynamic polarization curve, for the evaluation of the corrosion resistance in aerated solution of NaCL at 3.5%, shows a better corrosion resistance of the gloss side (the corrosion resistance is estimated at 214 millimicrons per year compared to 641 of the matt side). This performance can be related to point “2”, ie the greater amount of oxygen (Al2O3) on the gloss side, leading to a higher protection.
C. Sinagra - F. Bravaccino
R&D Laminazione Sottile SpA
C. Velotti
Università Federico II Napoli. DIMP




















