Pharmaceutical traceability: when "integration" means "integrity"
To offer a comprehensive response to the authenticity, integrity and security needs of the pharmaceutical industry, Weber Marking Systemsand Retinae have collaborated to develop a complete solution for serializing, sealing and branding pharmaceutical cartons. Centralization of functions is combined with a small device footprint in a state-of-the-art integrated system.

The supply chain of pharmaceutical products is becoming increasingly global, with end users and the distribution chain often located in countries other than the country of production. In this context, the increasing complexity of logistics is combined with the need to ensure an "end-to-end" traceability (from producer to consumer) of products, in order to eliminate the risks of theft and tampering.
In order to combat the phenomenon of counterfeit medicines, many countries have adopted stringent regulations imposing mandatory serialization and traceability of packaging. In Europe, the Falsified Medicines Directive has been in force for more than two years, and in Italy many companies are still looking for reliable solutions to serialize and secure their products.
Pharmaceutical companies and packaging machine manufacturers are, therefore, called to meet these needs, offering solutions that comply with the requirements of a highly regulated context.
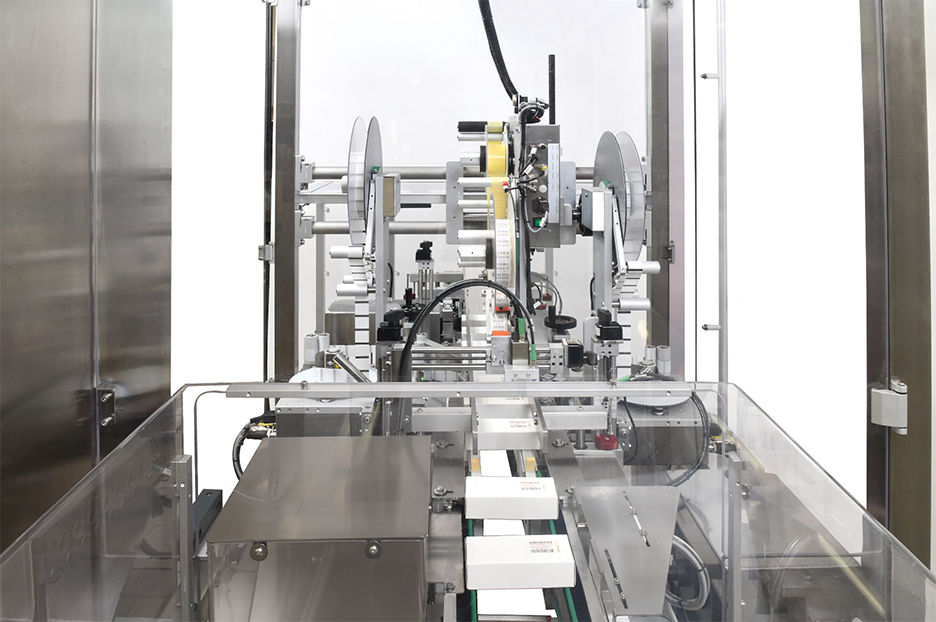
Serializing, sealing and labeling: an integrated process
A complete, state-of-the-art response to the needs of the pharmaceutical industry is offered by the new system developed by Retinæ, a specialist in machine vision and serialization solutions (up to level 3), in partnership with Weber Marking Systems, a leading manufacturer of innovative, high-performance solutions for industrial labeling.
This solution uses Retinæ's new CS150 automatic system for serializing and sealing pharmaceutical cartons, based on the modular "Print Verify & Serialize" technology, which allows integration on new machines, as well as revamping of cartoners or labelers, to implement serialization on existing lines. All labeling, coding and inspection devices are centrally managed from the system interface, so as to offer a simple and intuitive user experience to the operators.

CS150 also features a state-of-the-art positive pushers (porters) conveyor system to ensure precise product handling, even at high speeds.
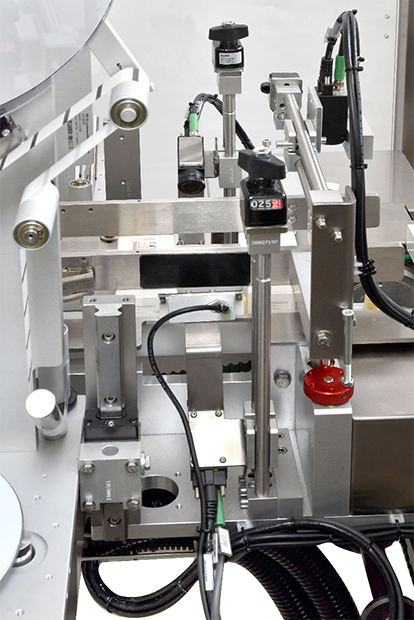 To implement labeling processes, Retinae has chosen automatic applicators by Weber Marking Systems.
To implement labeling processes, Retinae has chosen automatic applicators by Weber Marking Systems.
In particular, two TE 122 labelling machines have been integrated to seal the flaps of the cartons and an automatic applicator to affix the Italian optical stamp on the upper surface of the products.
One of the strengths of this solution lies in the extremely compact size of the system: the serialization, stamping, sealing and rejection functions are carried out in a length of just 1,800 mm.
Luca Bertocchi, Partner of Retinæ, says: «The collaboration with Weber has allowed us to offer our customers reliable labeling solutions, designed for very high productivity industrial environments, with the possibility of controlling every single parameter from our centralized management system.
We chose Weber as a partner because we appreciate their great experience in pharmaceutical track & trace applications. In particular, the ultra-compact tamper evident seal applicator represents a significant added value for the reduction of the system footprint».
SYSTEM STRENGTHS
Print Verify & Serialize system by Retinæ.
• Modular system that includes the possibility to control every single device of the line (automation, labeling, thermal inkjet printing and vision).
• Centralized management of all parameters through the creation of recipes for each individual format.
• System complies with CFR 21 requirements (access control via password, user rights for each level, integrity of files containing electronic documents, accurately recorded audit trails, electronic signatures).
• Easy diagnosis of each individual device, managed centrally by the system.
• Possibility of integrating the serialization solution directly on cartoning machines, even on pre-existing lines.
• Possibility of connection with Retinae level 3 "FSM", or alternatively with third party L3.
• Execution of Qualification Protocols (IQ-OQ-PQ).
Weber Marking Systems' evident tamper applicators
• Solution designed to apply tamper evident labels to the sides of pharmaceutical cartons.
• Extremely compact system with vertical development.
• Very simple paper passage for quick and intuitive format changeover.
• The label handling system on the rollers is powered by two separate stepper motors (puller and rewinder), so as to guarantee a constant tension of the label roll without the need for any mechanical adjustment.
• Possibility of remote control of each single parameter of the labeller through I/O connector or alternatively through commands in machine language.
Ready for the next step: aggregation
If, at present, the serialization obligation in Europe concerns only the unit pack, nevertheless the unambiguous identification of the tertiary and transport packaging of pharmaceutical products (bundles, boxes containing several unit packs, pallets) is required in other countries.
Even where it is not imposed at a regulatory level, this additional level of serialization - called "aggregation" - facilitates traceability throughout the supply chain up to the point of sale, contributing to the security of the supply chain. It also enables efficient returns management, eliminating the need to scan each individual unit.
Also in applications of this type, the collaboration between Retinæ and Weber Marking Systems enables Retinæ to offer Italian pharmaceutical manufacturers a complete solution for aggregating products along the entire packaging line, ensuring optimal management between the various packaging levels.
One of the strengths of this proposition is represented by the new Weber systems for printing and applying labels in real time of the Legi-Air 4050 Be Electric range: the use of electric applicators guarantees operator safety, process reliability and precision.
A value-added solution for the pharmaceutical industry
The total integration of technologically advanced devices in a single centralized system - the result of a joint effort between two companies of excellence - ensures a more reliable and safe management.
Last but not least, the solution is ready to be quickly integrated into any pharmaceutical packaging line.



















